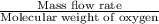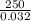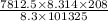Chemistry, 03.10.2019 02:00 alannaswitzer
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to estimate the volumetric flow rate (l/hr) of this stream. (see example 5.3-3.) the ideal gas equation of state is an approximation. under which conditions, it is suggested that the ideal gas equation be used for? select one: o a. temperatures above about 0°c and pressures below about 1 atm o b. temperatures below about 0°c and pressures below about 1 atm c. temperatures above about 0°c and pressures above about 1 atm d. under any condition e. standard condition of 25°c and 1 atm o o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1


You know the right answer?
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to...
Questions


Social Studies, 14.10.2019 23:00



Mathematics, 14.10.2019 23:00

English, 14.10.2019 23:00


English, 14.10.2019 23:00

Physics, 14.10.2019 23:00




Physics, 14.10.2019 23:00

Physics, 14.10.2019 23:00