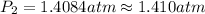Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
The vapor pressure of water is 1.00 atm at 373 k, and the enthalpy of vaporization is 40.68 kj mol!...
Questions

History, 11.06.2020 21:57



Mathematics, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57

Physics, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57



History, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57


History, 11.06.2020 21:57


Mathematics, 11.06.2020 21:57


History, 11.06.2020 21:57


Mathematics, 11.06.2020 21:57

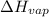 of the reaction, we use clausius claypron equation, which is:
of the reaction, we use clausius claypron equation, which is:![\ln(\frac{P_2}{P_1})=\frac{\Delta H_{vap}}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0284/2756/b9a49.png)
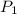 = vapor pressure at temperature
= vapor pressure at temperature 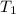
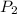 = vapor pressure at temperature
= vapor pressure at temperature 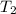
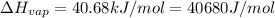
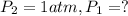
![\ln(\frac{1 atm}{P_1})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{363}-\frac{1}{373}]](/tpl/images/0284/2756/cf798.png)
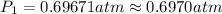
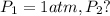
![\ln(\frac{P_2}{1 atm})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{383}]](/tpl/images/0284/2756/01f21.png)