
Chemistry, 02.10.2019 22:00 TropicalFan
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in hz) and wavelength (in nm) of one of these photons.
frequency hz
and
wavelength nm
what is the total energy (in kj) in 1 mole of these photons?
kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in h...
Questions

English, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

English, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

Chemistry, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40





Mathematics, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40


Mathematics, 25.02.2021 22:40

Mathematics, 25.02.2021 22:40



Advanced Placement (AP), 25.02.2021 22:40

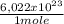 = 230040 J/mole ≡ 230,0 kJ/mole
= 230040 J/mole ≡ 230,0 kJ/mole

