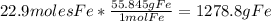Chemistry, 02.10.2019 21:20 fancycar14
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the balanced equation for this reaction? (use the lowest possible coefficients. omit states of matter.) b. what number of moles of iron reacts with 17.15 mol of oxygen from the air? mol c. what mass of iron is required to react with 17.15 mol of oxygen? mass =

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the b...
Questions


Mathematics, 09.10.2019 18:00






History, 09.10.2019 18:00

Mathematics, 09.10.2019 18:00

English, 09.10.2019 18:00

World Languages, 09.10.2019 18:00


Chemistry, 09.10.2019 18:00

Social Studies, 09.10.2019 18:00

Mathematics, 09.10.2019 18:00


Social Studies, 09.10.2019 18:00


History, 09.10.2019 18:00

Social Studies, 09.10.2019 18:00

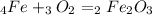
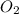
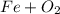
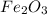 , so you should write the product:
, so you should write the product: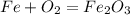
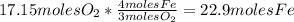
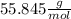 , so:
, so: