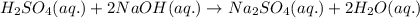Chemistry, 02.10.2019 21:20 tareas7009
When balancing the equation h2so4 (aq) + naoh (aq) → na2so4(aq) + h2o(aq), would you change the coefficients of the compounds or the subscripts of the compounds? explain your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3
You know the right answer?
When balancing the equation h2so4 (aq) + naoh (aq) → na2so4(aq) + h2o(aq), would you change the coef...
Questions

Advanced Placement (AP), 19.10.2020 04:01

Biology, 19.10.2020 04:01

Chemistry, 19.10.2020 04:01

History, 19.10.2020 04:01

Social Studies, 19.10.2020 04:01

Health, 19.10.2020 04:01


Mathematics, 19.10.2020 04:01





Social Studies, 19.10.2020 04:01

History, 19.10.2020 04:01





Mathematics, 19.10.2020 04:01

Mathematics, 19.10.2020 04:01