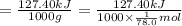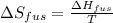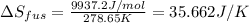Chemistry, 02.10.2019 21:10 CameronVand21
The enthalpy of fusion for benzene (c6h6, 78.0 g/mol) is 127.40 kj/kg, and its melting point is 5.5°c. what is the entropy change when 1 mole of benzene melts at 5.5°c? (show all unit conversions and write out all equations)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
The enthalpy of fusion for benzene (c6h6, 78.0 g/mol) is 127.40 kj/kg, and its melting point is 5.5°...
Questions












Mathematics, 12.04.2021 21:30

Mathematics, 12.04.2021 21:30

Mathematics, 12.04.2021 21:30



History, 12.04.2021 21:30

Mathematics, 12.04.2021 21:30


Mathematics, 12.04.2021 21:30

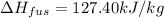
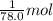
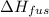 = 127.40 kJ/kg=127.40 kJ/1000 g (1kg = 1000 g)
= 127.40 kJ/kg=127.40 kJ/1000 g (1kg = 1000 g)