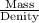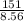Density i
mass
volume of metal
final water volume
step 1
use the metal’s density as a conversion factor to convert from mass (in units of grams) to volume (in units of milliliters).
step 2
the metal displaces its volume in the water so the water level rises by that volume. the final volume is determined by adding the initial volume and the volume of the metal.
you have a 100.0-ml graduated cylinder containing 50.0 ml of water. you carefully place a 151-g piece of brass (density = 8.56 g/ml) into the water. what is the final volume reading in the graduated cylinder?
ml
what is the volume that 151 g of brass occupies?
ml
if the brass displaces this volume of water, what is the final volume in the cylinder?
volume brass = 17.6 ml
ml

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Density i
mass
volume of metal
final water volume
st...
mass
volume of metal
final water volume
st...
Questions



Mathematics, 05.05.2020 19:43





History, 05.05.2020 19:43

Social Studies, 05.05.2020 19:43


Geography, 05.05.2020 19:43

Mathematics, 05.05.2020 19:43



Mathematics, 05.05.2020 19:43





Health, 05.05.2020 19:43