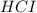Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn i...
Questions

Mathematics, 18.08.2019 18:50


Social Studies, 18.08.2019 18:50







Chemistry, 18.08.2019 18:50

Mathematics, 18.08.2019 18:50



Mathematics, 18.08.2019 18:50


Geography, 18.08.2019 18:50



Physics, 18.08.2019 18:50

History, 18.08.2019 18:50

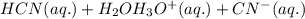
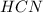 : acid
: acid 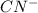 :conjugate base.
:conjugate base.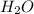 : base
: base 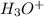 : conjugate acid.
: conjugate acid.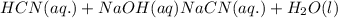
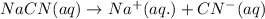
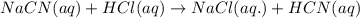
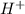 ions in their aqueous states.
ions in their aqueous states.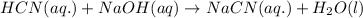
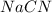 in water.
in water.