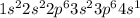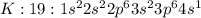Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
There is an element x ( atomic no. 19, atomic mass 40) (10) (a)what is its correct ground state orbi...
Questions

Advanced Placement (AP), 20.01.2021 21:10

Mathematics, 20.01.2021 21:10

History, 20.01.2021 21:10

Biology, 20.01.2021 21:10

Mathematics, 20.01.2021 21:10


Mathematics, 20.01.2021 21:10


Mathematics, 20.01.2021 21:10

Spanish, 20.01.2021 21:10









English, 20.01.2021 21:10

Mathematics, 20.01.2021 21:10