
Chemistry, 02.10.2019 03:00 bowmanari2154
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. 2-butanone 1-bromopropane magnesium 3-methyl-3-hexanol =0.81 g/ml =1.35 g/ml =0.82 g/ml a reaction was performed in which 0.40 ml0.40 ml of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.38 g0.38 g of 3‑methyl‑3‑hexanol. calculate the theoretical yield and percent yield for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1



Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made...
Questions

Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


Biology, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00

English, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00



History, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00



Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

English, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

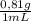 ×
×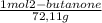 ×
×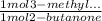 ×
×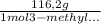 = 0,52 g
= 0,52 g

