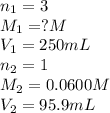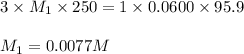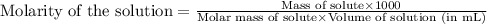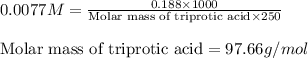Chemistry, 02.10.2019 02:00 Trevon0906
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask and dilutes to the mark with distilled water. he then titrates this solution with 0.0600 m naoh solutions. when the titration reaches the equivalence point, the chemist finds he has added 95.9 ml of naoh solution.
calculate the molar mass of the unknown acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask...
Questions





Biology, 26.07.2019 07:00


Social Studies, 26.07.2019 07:00


Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00


Physics, 26.07.2019 07:00

Biology, 26.07.2019 07:00


Chemistry, 26.07.2019 07:00

Biology, 26.07.2019 07:00

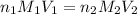
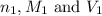 are the n-factor, molarity and volume of triprotic acid
are the n-factor, molarity and volume of triprotic acid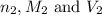 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.