Chemistry, 01.10.2019 22:20 savannahckatz
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891 g . the two rings are heated to 66.887 oc and dropped into a 13.9 ml of water at 21.9 oc. when equilibrium is reached, the temperature of the water is 24.2 oc. what is the mass of gold ring? (assume a density of 0.998 g/ml for water.)

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1
You know the right answer?
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891...
Questions

Business, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01

Physics, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01

Geography, 20.10.2020 23:01

Chemistry, 20.10.2020 23:01






Spanish, 20.10.2020 23:01





Mathematics, 20.10.2020 23:01

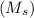 + mass of gold ring
+ mass of gold ring 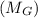
![[M_{G} \times 0.128 J/g^{o}C \times (66.887 - 24.2)^{o}C] + [M_{s} \times 0.235 J/g^{o}C \times (66.887 - 24.2)^{o}C]](/tpl/images/0281/0300/1110a.png) =
= 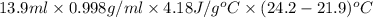
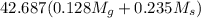 = 133.368
= 133.368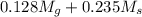 = 3.124
= 3.124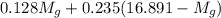 = 3.124
= 3.124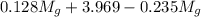 = 3.124
= 3.124 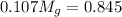
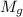 = 7.897 g
= 7.897 g