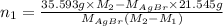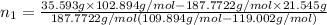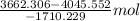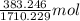Chemistry, 01.10.2019 22:00 GodlyGamer8239
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g mixture of kbr and nabr is dissolved in water, then a solution of agno3 is added so that all of the bromine present is converted to solid agbr. the agbr product is dried and found to have a mass of 35.593 g. what mass of kbr was present in the original mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g m...
Questions

English, 08.05.2021 09:30

Mathematics, 08.05.2021 09:30



Chemistry, 08.05.2021 09:30

Physics, 08.05.2021 09:30

Mathematics, 08.05.2021 09:30

Mathematics, 08.05.2021 09:30

Mathematics, 08.05.2021 09:30

Mathematics, 08.05.2021 09:30



Biology, 08.05.2021 09:30




Chemistry, 08.05.2021 09:30

English, 08.05.2021 09:30


Mathematics, 08.05.2021 09:30

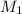 and NaBr is
and NaBr is 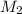 .
.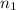 and
and 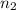 .
.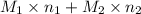 = 21.545 g
= 21.545 g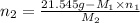
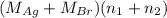 = 35.593 g
= 35.593 g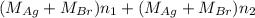 = 35.593 g
= 35.593 g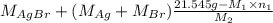 = 35.593 g
= 35.593 g