
Chemistry, 01.10.2019 21:10 ofcitsnijah
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture is heated in the presence of excess cl2, all of the kbr is converted to kcl. if the total mass of kcl present after this reaction is 3.129 g, what percentage (by mass) of the original mixture was kbr? (hint: be sure that you understand why the mass of the sample has decreased. it may if you write an equation for the reaction that converted the kbr to kcl.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture...
Questions

Mathematics, 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00



English, 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00


Biology, 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00


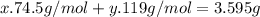
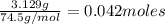
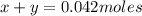
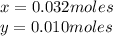
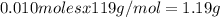
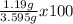 %
% 

