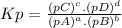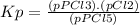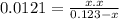Chemistry, 01.10.2019 19:00 sierravick123owr441
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl 3 (g) cl 2 (g) a vessel is charged with pcl 5, giving an initial pressure of 0.123 atm. at equilibrium, the partial pressure of pcl 3 is atm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl...
Questions

Mathematics, 23.02.2021 14:00



Mathematics, 23.02.2021 14:00

History, 23.02.2021 14:00

History, 23.02.2021 14:00

English, 23.02.2021 14:00


History, 23.02.2021 14:00

Computers and Technology, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Chemistry, 23.02.2021 14:00

Chemistry, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00


History, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

![Kc = \frac{[C]^c.[D]^d}{[A]^a.[B]^b}](/tpl/images/0280/5110/4ea0c.png)