
Chemistry, 01.10.2019 19:00 F00Dislife
Consider the equation: 2no(0) - n. using only the information given by the equation which of the following
changes would increase the molar concentration at equilibrium of the product n, o4(0)?
1)increase the temperature
2)decrease the pressure
3)decrease the temperature
4)decrease the concentration of no ala)
5)increase the pressure

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Consider the equation: 2no(0) - n. using only the information given by the equation which of the fo...
Questions



Mathematics, 04.01.2020 00:31

Physics, 04.01.2020 00:31

History, 04.01.2020 00:31

Mathematics, 04.01.2020 00:31


Computers and Technology, 04.01.2020 00:31

Chemistry, 04.01.2020 00:31





Mathematics, 04.01.2020 00:31

Advanced Placement (AP), 04.01.2020 00:31





History, 04.01.2020 00:31

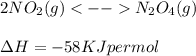

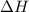 is not given
is not given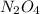 is not produced in greater amount.
is not produced in greater amount.


