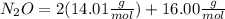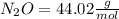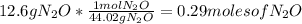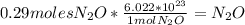Chemistry, 01.10.2019 17:30 arianawelsh123l
Dinitrogen monoxide or laughing gas (n2o) is used as a dental anesthetic and as an aerosol propellant. how many moles of n2o are present in 12.6 g of the compound? how many molecules of n2o are present in 12.6 g of the compound? [use molar masses: n, 14.01 g/mol, o, 16.00 g/mol]

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Dinitrogen monoxide or laughing gas (n2o) is used as a dental anesthetic and as an aerosol propellan...
Questions

World Languages, 02.06.2021 04:00


Biology, 02.06.2021 04:00



Biology, 02.06.2021 04:00

Chemistry, 02.06.2021 04:00


Law, 02.06.2021 04:00

Biology, 02.06.2021 04:00



Geography, 02.06.2021 04:00

Mathematics, 02.06.2021 04:00


Mathematics, 02.06.2021 04:00


Mathematics, 02.06.2021 04:00

Mathematics, 02.06.2021 04:00

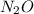 there are 0.29 moles of
there are 0.29 moles of 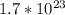 molecules of
molecules of