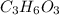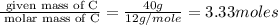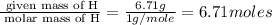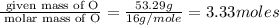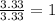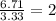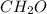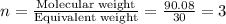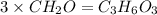Chemistry, 01.10.2019 17:30 MansellS5529
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in carbon, 6.71% mass in hydrogen, and the remaining mass in oxygen. determine its empirical formula. the formula mass of the unknown is independently determined to be 90.08 g/mol, determine the unknown’s molecular formula

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in car...
Questions

Chemistry, 30.08.2019 23:30


Mathematics, 30.08.2019 23:30



Biology, 30.08.2019 23:30

History, 30.08.2019 23:30

Health, 30.08.2019 23:30



Health, 30.08.2019 23:30

History, 30.08.2019 23:30


Computers and Technology, 30.08.2019 23:30