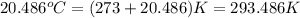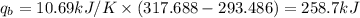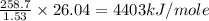Chemistry, 01.10.2019 17:20 mauifrifer3986
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. when 1.53 g of acetylene (c2h2) is burned in a bomb calorimeter with a heat capacity of 10.69 kj/k, the temperature increases from 20.486°c to 44.688°c. what is δe (in kj/mol) for this combustion reaction? enter to 0 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1


Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1
You know the right answer?
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. w...
Questions

Mathematics, 17.04.2021 23:10

Chemistry, 17.04.2021 23:10



Chemistry, 17.04.2021 23:10

Spanish, 17.04.2021 23:10

Mathematics, 17.04.2021 23:10

Biology, 17.04.2021 23:10


Chemistry, 17.04.2021 23:10



Mathematics, 17.04.2021 23:10

Chemistry, 17.04.2021 23:10

Arts, 17.04.2021 23:10


Chemistry, 17.04.2021 23:10


SAT, 17.04.2021 23:10

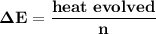
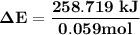
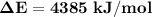
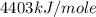
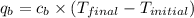
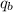 = heat absorbed by calorimeter = ?
= heat absorbed by calorimeter = ?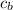 = specific heat of = 10.69 kJ/K
= specific heat of = 10.69 kJ/K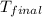 = final temperature =
= final temperature = 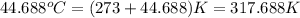
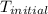 = initial temperature =
= initial temperature =