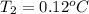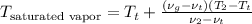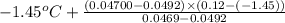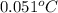Aclosed, rigid tank contains a two‐phase liquid–vapor mixture of refrigerant 22 initially at −20°c with a quality of 50.36%. energy transfer by heat into the tank occurs until the refrigerant is at a final pressure of 6 bar. determine the final temperature, in °c. if the final state is in the superheated vapor region, at what temperature, in °c, does the tank contain only saturated vapor?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Aclosed, rigid tank contains a two‐phase liquid–vapor mixture of refrigerant 22 initially at −20°c w...
Questions

Social Studies, 26.09.2019 18:40

Mathematics, 26.09.2019 18:40

Mathematics, 26.09.2019 18:40

Chemistry, 26.09.2019 18:40




Mathematics, 26.09.2019 18:40






Mathematics, 26.09.2019 18:40


Mathematics, 26.09.2019 18:40


English, 26.09.2019 18:40


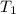 ) =
) = 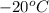
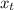 ) = 0.5036
) = 0.5036 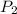 ) = 6 bar
) = 6 bar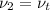
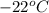 is as follows.
is as follows.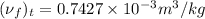
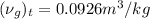
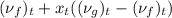
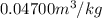
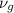 =
= 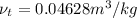 and
and 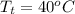
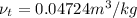 and
and 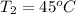
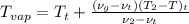
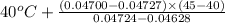
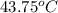
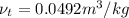 and
and 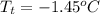
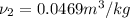 and
and