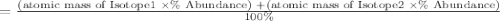Chemistry, 01.10.2019 06:30 trentvw1174
Afictitious element q has two naturally occurring isotopes. the first isotope has an abundance of 20.00% and a mass of 39.96 amu and the second isotope of element q has an abundance of 80.00% and a mass of 43.39amu. calculate the weighted atomic mass of element q to the nearest tenth. to earn credit, be sure to show the work that leads to your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1


Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
Afictitious element q has two naturally occurring isotopes. the first isotope has an abundance of 20...
Questions



Mathematics, 30.08.2019 15:10


History, 30.08.2019 15:10

Mathematics, 30.08.2019 15:10



History, 30.08.2019 15:10





Mathematics, 30.08.2019 15:10

Physics, 30.08.2019 15:10


Mathematics, 30.08.2019 15:10

Chemistry, 30.08.2019 15:10