
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a capacity of 300.0 l and is filled with 35.0 atm of helium gas. room a has a capacity of 200.0 l and holds 20.0 atm of nitrogen gas. room b has 40.0 atm of oxygen gas in 180.0 l. a. if you open both rooms to the hallway, what would be pressure of each gas in the combined space of the hallway and two rooms? b. what would be the mol fraction of oxygen in that gas? c. after mixing the gases, if you let gas escape until the total pressure in the hallway was 1.2 atm, what would be the final partial pressure of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a cap...
Questions



Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31



Computers and Technology, 24.01.2020 22:31



Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31

History, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31




Mathematics, 24.01.2020 22:31



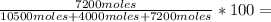 33,2% O₂
33,2% O₂

