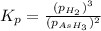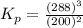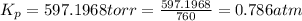Chemistry, 01.10.2019 04:20 gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions

Mathematics, 05.01.2020 01:31







Physics, 05.01.2020 01:31






Mathematics, 05.01.2020 01:31

Chemistry, 05.01.2020 01:31

History, 05.01.2020 01:31

English, 05.01.2020 01:31

Mathematics, 05.01.2020 01:31

Law, 05.01.2020 01:31

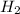 gas is, 288 torr
gas is, 288 torr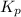 for this reaction is, 0.786 atm
for this reaction is, 0.786 atm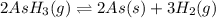
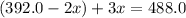
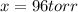
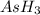 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr