
Chemistry, 01.10.2019 04:10 bettybales1986
A26.08 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. the reaction mixture is then reacted with 132 ml of 3.80 m barium chloride to produce the maximum possible amount of barium sulfate. determine the percent sodium by mass in the original mixture.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
A26.08 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. the re...
Questions




Biology, 24.01.2021 04:10

Mathematics, 24.01.2021 04:10


Mathematics, 24.01.2021 04:10

Mathematics, 24.01.2021 04:10

English, 24.01.2021 04:10


English, 24.01.2021 04:10

Mathematics, 24.01.2021 04:10

Mathematics, 24.01.2021 04:20



Business, 24.01.2021 04:20


Mathematics, 24.01.2021 04:20

Chemistry, 24.01.2021 04:20

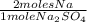 × 22,99 g/mole = 23,08 g of Na
× 22,99 g/mole = 23,08 g of Na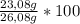 = 88,50%
= 88,50% 


