Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs...

Chemistry, 19.08.2019 10:30 terryonsavage543
Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs when exactly1 mol of a compound is formed from its constituent elements under standard conditions. the standard conditions are 1 atm pressure, a temperature of 25 ∘c , and all the species present at a concentration of 1 m . a "standard enthalpies of formation table" containing δh∘f values might look something like this: substanceδh∘fh(g)218 kj/molh2(g)0 kj/molba(s)0 kj/molba2+(aq)−538.4 kj/molc(g)71 kj/molc(s)0 kj/moln(g)473 kj/molo2(g)0 kj/molo(g)249 kj/mols2(g)129 kj/mol
question:
what is the balanced chemical equation for the reaction used to calculate δh∘f of baco3(s)? if fractional coefficients are required, enter them as a fraction (i. e. 1/3). indicate the physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. use (aq) for aqueous solution.
express answer as a chemical equation. explain for me !

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Questions

Biology, 05.12.2021 04:50



Mathematics, 05.12.2021 04:50

Mathematics, 05.12.2021 04:50

Biology, 05.12.2021 04:50

History, 05.12.2021 04:50

Mathematics, 05.12.2021 04:50

Advanced Placement (AP), 05.12.2021 04:50

Biology, 05.12.2021 04:50


Mathematics, 05.12.2021 04:50


Mathematics, 05.12.2021 04:50

Mathematics, 05.12.2021 04:50

Physics, 05.12.2021 04:50

Mathematics, 05.12.2021 04:50


Mathematics, 05.12.2021 05:00

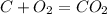
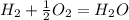
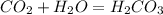
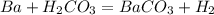
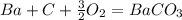 , using hydrogen as a catalyst.
, using hydrogen as a catalyst.

