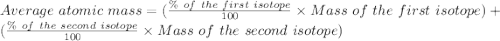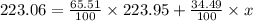Chemistry, 01.10.2019 00:30 20guadalupee73248
Afictitious element z has an average atomic mass of 223.06 u.223.06 u. element z has two naturally occuring isotopes. the more abundant isotope has an exact mass of 223.95 u223.95 u and a relative abundance of 65.51%.65.51%. calculate the exact mass of the second isotope.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1
You know the right answer?
Afictitious element z has an average atomic mass of 223.06 u.223.06 u. element z has two naturally o...
Questions





Mathematics, 05.08.2020 16:01







Social Studies, 05.08.2020 16:01

Biology, 05.08.2020 16:01