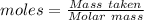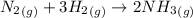Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1



You know the right answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions

Chemistry, 07.05.2021 04:20



Mathematics, 07.05.2021 04:20

Chemistry, 07.05.2021 04:20


Mathematics, 07.05.2021 04:20

Mathematics, 07.05.2021 04:20

Mathematics, 07.05.2021 04:20


Mathematics, 07.05.2021 04:20


Mathematics, 07.05.2021 04:20


Mathematics, 07.05.2021 04:20


Mathematics, 07.05.2021 04:20



 .
.