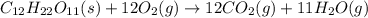Chemistry, 30.09.2019 21:20 monnn91351
3. the chemical formula of a compound does not indicate
the identity of the elements in the compound
how elements are joined in the compound
the composition of the compound
the relative proportions of the elements in the compound
4. in the chemical reaction in which sucrose is heated and decomposes to form carbon dioxide and water,
which of the following is a reactant?
sucrose
carbon dioxide
water
heat
5. which of the following is not a physical change?
grating cheese
melting cheese
fermenting of cheese
mixing two cheeses in a bowl
6. a chemical change occurs when a piece of wood
is split
is painted
decays
is cut
7. which of the following does not indicate that a chemical change may have taken place?
fracture formation
gas production
precipitate formation
energy transfer
8. what happens to matter during a chemical reaction?
matter is neither destroyed or created.
some matter is destroyed.
some matter is created.
some matter is destroyed and some is created.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
3. the chemical formula of a compound does not indicate
the identity of the elements in the...
the identity of the elements in the...
Questions



Mathematics, 15.07.2019 07:00



History, 15.07.2019 07:00



Business, 15.07.2019 07:00


History, 15.07.2019 07:00

Mathematics, 15.07.2019 07:00




Biology, 15.07.2019 07:00

Business, 15.07.2019 07:00



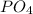 .
.