
Chemistry, 30.09.2019 20:30 poweradampower
2n2h4(l) + n2o4(l) → 3n2(g) + 4h2o(g) [balanced] how many moles of n2h4 is required to produce 28.3 g of n2? assume that all reactants react completely. molar mass of n2h4 = 32.06 g/mol molar mass of n2o4 = 92.02 g/mol molar mass of n2 = 28.02 g/mol molar mass of h2o = 18.02 g/mol group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
2n2h4(l) + n2o4(l) → 3n2(g) + 4h2o(g) [balanced] how many moles of n2h4 is required to produce 28.3...
Questions

Mathematics, 05.12.2019 18:31

Mathematics, 05.12.2019 18:31



History, 05.12.2019 18:31

Mathematics, 05.12.2019 19:31

Biology, 05.12.2019 19:31


History, 05.12.2019 19:31

Chemistry, 05.12.2019 19:31



Mathematics, 05.12.2019 19:31


History, 05.12.2019 19:31





Chemistry, 05.12.2019 19:31

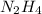
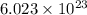 of particles.
of particles.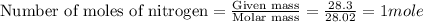
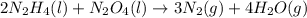
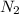 is produced by 2 moles of
is produced by 2 moles of 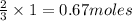 of
of 

