
Chemistry, 28.09.2019 05:30 negritoboy014
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tumsr is 40.0 percent caco₃ by mass. using only tumsr, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions





Health, 12.10.2020 04:01





English, 12.10.2020 04:01


Social Studies, 12.10.2020 04:01







Mathematics, 12.10.2020 04:01

History, 12.10.2020 04:01

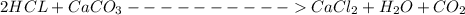
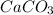 and HCl is 1 : 2
and HCl is 1 : 2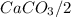 mol HCl = 0.1 mol
mol HCl = 0.1 mol 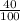 = 0.4
= 0.4 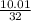 = 0.3128125 (number of containers)
= 0.3128125 (number of containers)

