
Chemistry, 28.09.2019 04:10 StephenCurry34
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k= 6.90 °c-kg-mol. calculate the freezing point of a solution made of 7.57g of sodium chloride (nacl) dissolved in 350. g of x round your answer to 3 significant digits. lºc x 5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1


Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k=...
Questions

Mathematics, 25.02.2020 21:55

Computers and Technology, 25.02.2020 21:55





Computers and Technology, 25.02.2020 21:55






History, 25.02.2020 21:56




Computers and Technology, 25.02.2020 21:56

Mathematics, 25.02.2020 21:56


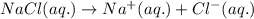
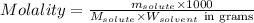
 = Given mass of solute (NaCl) = 7.57 g
= Given mass of solute (NaCl) = 7.57 g = Molar mass of solute (NaCl) = 58.44 g/mol
= Molar mass of solute (NaCl) = 58.44 g/mol = Mass of solvent (liquid X) = 350.0 g
= Mass of solvent (liquid X) = 350.0 g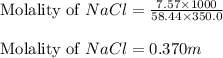
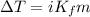
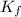 = molal freezing point depression constant = 6.90°C/m
= molal freezing point depression constant = 6.90°C/m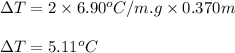
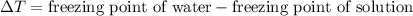
 = 5.11 °C
= 5.11 °C



