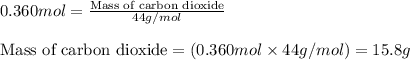Be sure to answer all parts. barium carbonate decomposes upon heating to barium oxide and carbon dioxide. enter and balance the equation (including the physical states) calculate the number of g of carbon dioxide produced by heating 71.0 g of barium carbonate. g co2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1


Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

You know the right answer?
Be sure to answer all parts. barium carbonate decomposes upon heating to barium oxide and carbon dio...
Questions

History, 13.11.2019 21:31




Mathematics, 13.11.2019 21:31



Mathematics, 13.11.2019 21:31



English, 13.11.2019 21:31


Geography, 13.11.2019 21:31

English, 13.11.2019 21:31

Computers and Technology, 13.11.2019 21:31



Biology, 13.11.2019 21:31


History, 13.11.2019 21:31

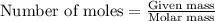 ......(1)
......(1)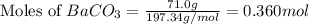
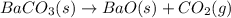
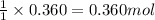 of carbon dioxide.
of carbon dioxide.