Chemistry, 28.09.2019 03:30 kyramks421
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alkane. termed hydrogenation, this type of reaction is used to produce products such as margarine. a typical hydrogenation reaction is c10h20() + h2(g) → c10h22(5) decene decane how much decane can be produced in a reaction of excess decene with 2.45 g hydrogen? give your answer in scientific notation. o *10 g decane

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1



Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alk...
Questions




Mathematics, 16.03.2022 07:40





SAT, 16.03.2022 07:40



Mathematics, 16.03.2022 07:50


Computers and Technology, 16.03.2022 07:50



English, 16.03.2022 07:50

Mathematics, 16.03.2022 07:50


Social Studies, 16.03.2022 07:50

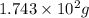
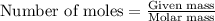 ......(1)
......(1)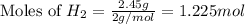
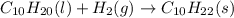
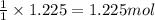 of decane
of decane