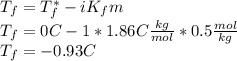Calculate the freezing temperature of the following solution of 0.50 m glucose (a covalent compound). assume that the molality of the solution is 0.50 m. (the molar and molal concentrations of dilute aqueous solutions are often identical to two significant figures.) enter your answer in the provided box. 0.50 m glucose (a covalent compound) °c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Calculate the freezing temperature of the following solution of 0.50 m glucose (a covalent compound)...
Questions





History, 26.03.2021 05:50


Mathematics, 26.03.2021 05:50


Mathematics, 26.03.2021 05:50



Advanced Placement (AP), 26.03.2021 05:50

Mathematics, 26.03.2021 05:50





Chemistry, 26.03.2021 05:50

Mathematics, 26.03.2021 05:50

Mathematics, 26.03.2021 05:50

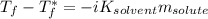
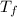 is the freezing temperature of the solution,
is the freezing temperature of the solution, 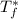 is the freezing temperature of the pure solvent (0 °C since it is water),
is the freezing temperature of the pure solvent (0 °C since it is water),  the Van't Hoff factor (1 since the solute is covalent),
the Van't Hoff factor (1 since the solute is covalent), 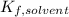 the solvent's freezing point depression point constant (in this case
the solvent's freezing point depression point constant (in this case 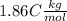 ) and
) and 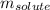 the molality of the glucose.
the molality of the glucose.