
Chemistry, 28.09.2019 03:10 Tonyang1742
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g), respectively, is 191.5, 205.0, & 240.5 j/mol-k -156.0 j/k 156.0 j/k 120.5 j/k -120.5 j/k o ooo

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g...
Questions


Social Studies, 30.05.2020 07:57


Mathematics, 30.05.2020 07:57

Mathematics, 30.05.2020 07:57



Mathematics, 30.05.2020 07:57



Mathematics, 30.05.2020 07:57

Mathematics, 30.05.2020 07:57


History, 30.05.2020 07:57


Mathematics, 30.05.2020 07:57

Mathematics, 30.05.2020 07:57

World Languages, 30.05.2020 07:57


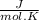 - [ 1 mol × 191,5
- [ 1 mol × 191,5 

