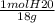Chemistry, 28.09.2019 03:10 marendt2014
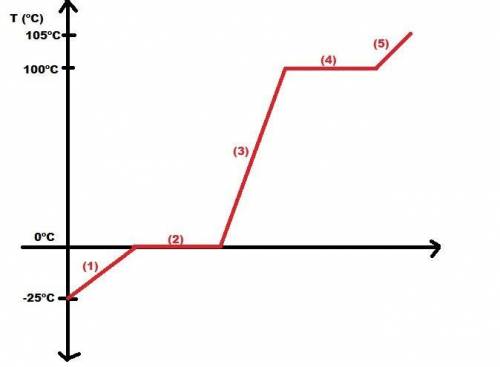
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a closed vessel and sketch a heating curve for the process. the specific heat of ice is 2.11 j/(g. "c); 4.18 j/g. "c) for water, 2.00 j/g. "c. ahus for water is 6,01 kj/mol; ahp for water = 40.67 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a...
Questions

World Languages, 25.07.2019 15:30




Biology, 25.07.2019 15:30

Mathematics, 25.07.2019 15:30

Mathematics, 25.07.2019 15:30

Social Studies, 25.07.2019 15:30



Health, 25.07.2019 15:30

Computers and Technology, 25.07.2019 15:30




Health, 25.07.2019 15:30

Health, 25.07.2019 15:30


English, 25.07.2019 15:30

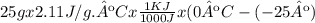
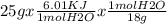
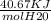 x
x