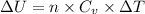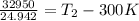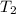Chemistry, 28.09.2019 02:20 IsabellaGracie
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j of heat are transferred to the gas, as a result of which the gas expands and does 1216 j of work against its surroundings. the process is reversible. (note: c = 1.5r) calculate the final temperature of the gas

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j o...
Questions

Physics, 21.06.2019 19:20

History, 21.06.2019 19:20


Spanish, 21.06.2019 19:30

Mathematics, 21.06.2019 19:30

Mathematics, 21.06.2019 19:30


Physics, 21.06.2019 19:30


Social Studies, 21.06.2019 19:30

Geography, 21.06.2019 19:30


Mathematics, 21.06.2019 19:30




Social Studies, 21.06.2019 19:30



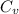 =
= 
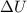 ) = Q + W
) = Q + W