Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
non-electrolyte = a, 10.6 grams, dissolved in solvent b, 740 grams, the boiling point of the solutio...
Questions

Mathematics, 23.01.2020 16:31

Physics, 23.01.2020 16:31



English, 23.01.2020 16:31




History, 23.01.2020 16:31

Biology, 23.01.2020 16:31



Mathematics, 23.01.2020 16:31

Mathematics, 23.01.2020 16:31


Social Studies, 23.01.2020 16:31


Mathematics, 23.01.2020 16:31


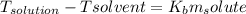
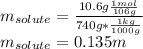
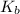 , we get:
, we get: