

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
If the amount of energy required to break bonds in the reactants is more than the amount of energy r...
Questions


Mathematics, 01.09.2019 17:30


Physics, 01.09.2019 17:30

Biology, 01.09.2019 17:30

Computers and Technology, 01.09.2019 17:30


English, 01.09.2019 17:30

History, 01.09.2019 17:30



English, 01.09.2019 17:30


History, 01.09.2019 17:30

Mathematics, 01.09.2019 17:30

Chemistry, 01.09.2019 17:30


History, 01.09.2019 17:30


English, 01.09.2019 17:30

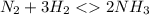
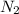 contains one N ≡ N triple bond (Bond breaking 946 KJ per mol)
contains one N ≡ N triple bond (Bond breaking 946 KJ per mol)
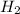 contains a single H-H bond (bond breaking 436KJ per mol)
contains a single H-H bond (bond breaking 436KJ per mol)
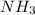 contains 3 N-H single bonds (389 KJ per mol)
contains 3 N-H single bonds (389 KJ per mol)


