
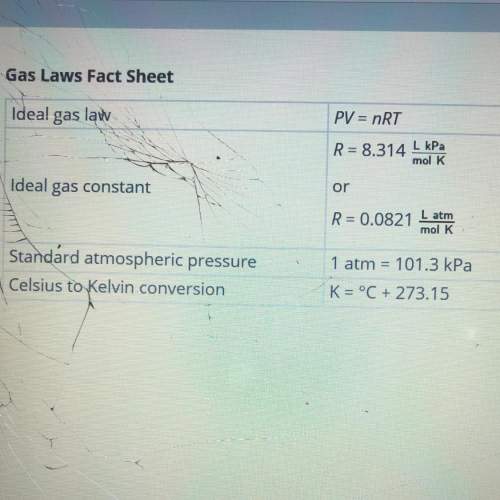
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at temperature of 495c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that t...
Questions





History, 03.07.2019 07:50



Mathematics, 03.07.2019 07:50





Biology, 03.07.2019 07:50


Biology, 03.07.2019 07:50

Mathematics, 03.07.2019 07:50






