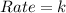Chemistry, 27.09.2019 06:00 kharmaculpepper
Be sure to answer all parts. determine the overall orders of the reactions to which the following rate laws apply: (a) rate = k[no2]2 (b) rate = k zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third ord

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
Be sure to answer all parts. determine the overall orders of the reactions to which the following ra...
Questions


English, 07.09.2021 07:20


English, 07.09.2021 07:20


Mathematics, 07.09.2021 07:20



Chemistry, 07.09.2021 07:20

Mathematics, 07.09.2021 07:20


Mathematics, 07.09.2021 07:20


Mathematics, 07.09.2021 07:20


History, 07.09.2021 07:20



Mathematics, 07.09.2021 07:20

![Rate=k[NO_2]^2](/tpl/images/0267/1831/d48ef.png)
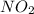 is 2.
is 2.