
Chemistry, 27.09.2019 01:10 maybeemmamay
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lithium nitride is g. molar mass of li is 6.94 g/mol. molar mass of n2 is 28.02 g/mol. molar mass of li3n is 34.83 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lit...
Questions

Biology, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

Chemistry, 09.12.2020 05:00

Advanced Placement (AP), 09.12.2020 05:00

Mathematics, 09.12.2020 05:00


Mathematics, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00


Health, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

English, 09.12.2020 05:00


Biology, 09.12.2020 05:00

Health, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

Computers and Technology, 09.12.2020 05:00

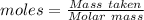
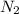
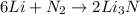
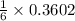 mole of
mole of 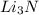
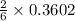 mole of
mole of 

