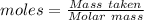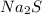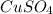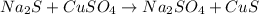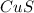What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s and 12.1 g cuso4? the actually amount of cus produced was 3.05 g. reaction: na2s + cuso4 → na2so4 + cus (a) 16.1% (b) 42.1% (c) 18.93% (d) 7.25% (e) not enough information

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

You know the right answer?
What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s...
Questions







Mathematics, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31

Mathematics, 10.12.2019 00:31

Biology, 10.12.2019 00:31