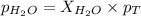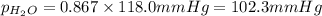Chemistry, 26.09.2019 19:00 Tyrant4life
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoch2ch2oh at 55 °c. the partial pressure of pure water at 55.0 °c is 118.0 mm hg. assume ideal behavior for the solution.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoc...
Questions

Mathematics, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30

History, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30





History, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30



History, 18.09.2019 08:30

History, 18.09.2019 08:30

History, 18.09.2019 08:30

Biology, 18.09.2019 08:30

Biology, 18.09.2019 08:30


English, 18.09.2019 08:30

History, 18.09.2019 08:30

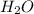 is 102.3 mmHg.
is 102.3 mmHg.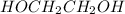 = 33.2 g
= 33.2 g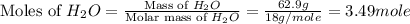
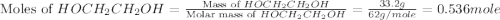
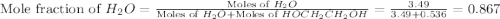
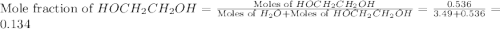
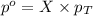
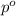 = partial pressure of water vapor
= partial pressure of water vapor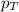 = total pressure of gas
= total pressure of gas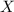 = mole fraction of water vapor
= mole fraction of water vapor