
Chemistry, 26.09.2019 18:00 zoeatlowapple
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation states, how many electrons would each sodium atom lose, and how many electrons would each fluorine atom gain? #e- each sodium atom would loose? #e- each fluorine atom would gain? how many sodium atoms are needed to react with one fluorine molecule?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation...
Questions

Computers and Technology, 19.04.2021 22:40



Health, 19.04.2021 22:40




Social Studies, 19.04.2021 22:40


Advanced Placement (AP), 19.04.2021 22:40




Social Studies, 19.04.2021 22:40

Advanced Placement (AP), 19.04.2021 22:40

Chemistry, 19.04.2021 22:40


History, 19.04.2021 22:40


![[Ne]3s^1](/tpl/images/0265/3273/4a392.png) .
.
![[He]2s^22p^5](/tpl/images/0265/3273/da8fe.png) .
.
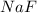 .
.

