
Be sure to answer all parts. consider the reaction a + b → products from the following data obtained at a certain temperature, determine the order of the reaction. enter the order with respect to a, the order with respect to b, and the overall reaction order. a 0.213 [a] (m) [b] (m) rate (m/s) 1.50 1.50 3.20 × 10−1 1.50 2.50 3.20 × 10−1 3.00 1.50 6.40 × 10−1 b 0.213 reaction 1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1
You know the right answer?
Be sure to answer all parts. consider the reaction a + b → products from the following data obtained...
Questions


History, 29.11.2019 18:31


Mathematics, 29.11.2019 18:31






English, 29.11.2019 18:31

Biology, 29.11.2019 18:31

Social Studies, 29.11.2019 18:31




Social Studies, 29.11.2019 18:31

English, 29.11.2019 18:31

Mathematics, 29.11.2019 18:31

Biology, 29.11.2019 18:31

Geography, 29.11.2019 18:31

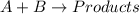
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0265/2010/10aeb.png)
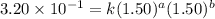 ....(1)
....(1)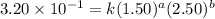 ....(2)
....(2)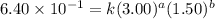 ....(3)
....(3)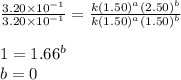
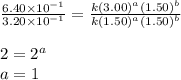
![\text{Rate}=k[A]^1[B]^0](/tpl/images/0265/2010/de6a4.png)
![\text{Rate}=k[A]](/tpl/images/0265/2010/660c6.png)


