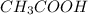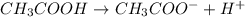Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?
a) weak electrolytes do not dissolve in water and can not conduct electricity.
b) weak electrolytes dissolve and partially dissociate in water providing charged ions to conduct electricity.
c) weak electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity.
d) weak electrolytes dissolve and does not dissociate in water providing no charged ions to conduct electricity.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 13:30
Which factors would have influenced earth’s climate during the time of pangea? check all that apply. land covered by glaciers one large landmass one large ocean tectonic activity multiple small seas
Answers: 2
You know the right answer?
Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?...
Questions




Biology, 26.04.2021 03:30

Engineering, 26.04.2021 03:30


Mathematics, 26.04.2021 03:30

Mathematics, 26.04.2021 03:30


English, 26.04.2021 03:30






Mathematics, 26.04.2021 03:30


Mathematics, 26.04.2021 03:30

Biology, 26.04.2021 03:30

Mathematics, 26.04.2021 03:30