Chemistry, 25.09.2019 02:01 hlgerardip4wbhx
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−4 g mgcl2 in 2.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−...
Questions

Mathematics, 25.11.2020 01:20

Advanced Placement (AP), 25.11.2020 01:20






Mathematics, 25.11.2020 01:20

Mathematics, 25.11.2020 01:30


Mathematics, 25.11.2020 01:30

Social Studies, 25.11.2020 01:30

Geography, 25.11.2020 01:30







English, 25.11.2020 01:30

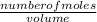
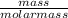
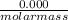 = 0.000252moles
= 0.000252moles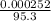 = 2.64 x 10⁻⁶moldm⁻³
= 2.64 x 10⁻⁶moldm⁻³