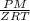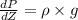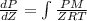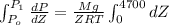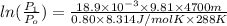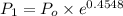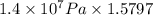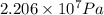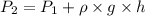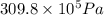Chemistry, 25.09.2019 02:30 YARETZYVENCES2144
Pressure gauge at the top of a vertical oil well registers 140 bars. the oil well is 6000 m deep and filled with natural gas down to a depth of 4700 m and filled with oil (density=700 kg/m3) the rest of the way to the bottom of the well at 15°c. the compressibility factor z=0.80 for natural gas and its molecular weight is 18.9.
determine the pressure at (a) the natural gas-oil interface and at (b) the bottom of the well at 15°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 21.06.2019 21:30
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
Pressure gauge at the top of a vertical oil well registers 140 bars. the oil well is 6000 m deep and...
Questions


Mathematics, 18.11.2020 04:50

Arts, 18.11.2020 04:50



Social Studies, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50




Chemistry, 18.11.2020 04:50


English, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50

Arts, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50

Health, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50

Mathematics, 18.11.2020 04:50

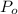 ) = 140 bar =
) = 140 bar = 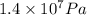 (as 1 bar =
(as 1 bar = 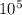 )
)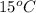 = (15 + 273) K = 288 K
= (15 + 273) K = 288 K