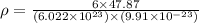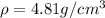Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
Titanium has an hcp unit cell for which the ratio of the lattice parameters cais 1.58. if the radius...
Questions



Mathematics, 26.01.2022 07:40




SAT, 26.01.2022 07:40


Chemistry, 26.01.2022 07:40

Computers and Technology, 26.01.2022 07:40

Mathematics, 26.01.2022 07:40


Mathematics, 26.01.2022 07:40







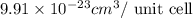
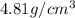
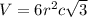
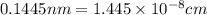
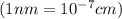
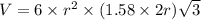
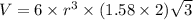
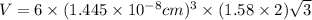
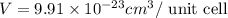
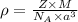
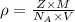 ..........(1)
..........(1)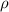 = density of Ti = ?
= density of Ti = ?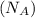 = Avogadro's number =
= Avogadro's number = 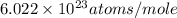
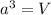 = volume of unit cell =
= volume of unit cell =